Ultrananaotech, ULTRANANOTECH
The Power of Catalysts: Driving Innovation in Electrochemical Devices
A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself being consumed during the reaction.
During a chemical reaction, the bonds between the atoms in molecules are broken, rearranged, and rebuilt, recombining the atoms into new molecules. Catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be surmounted for a chemical reaction to occur. As a result, catalysts make it easier for atoms to break and form chemical bonds to produce new combinations and new substances.
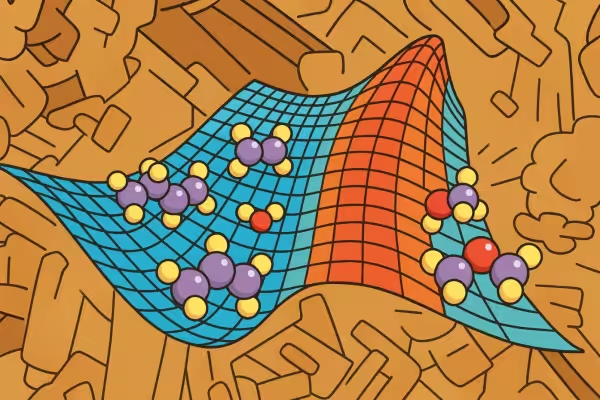
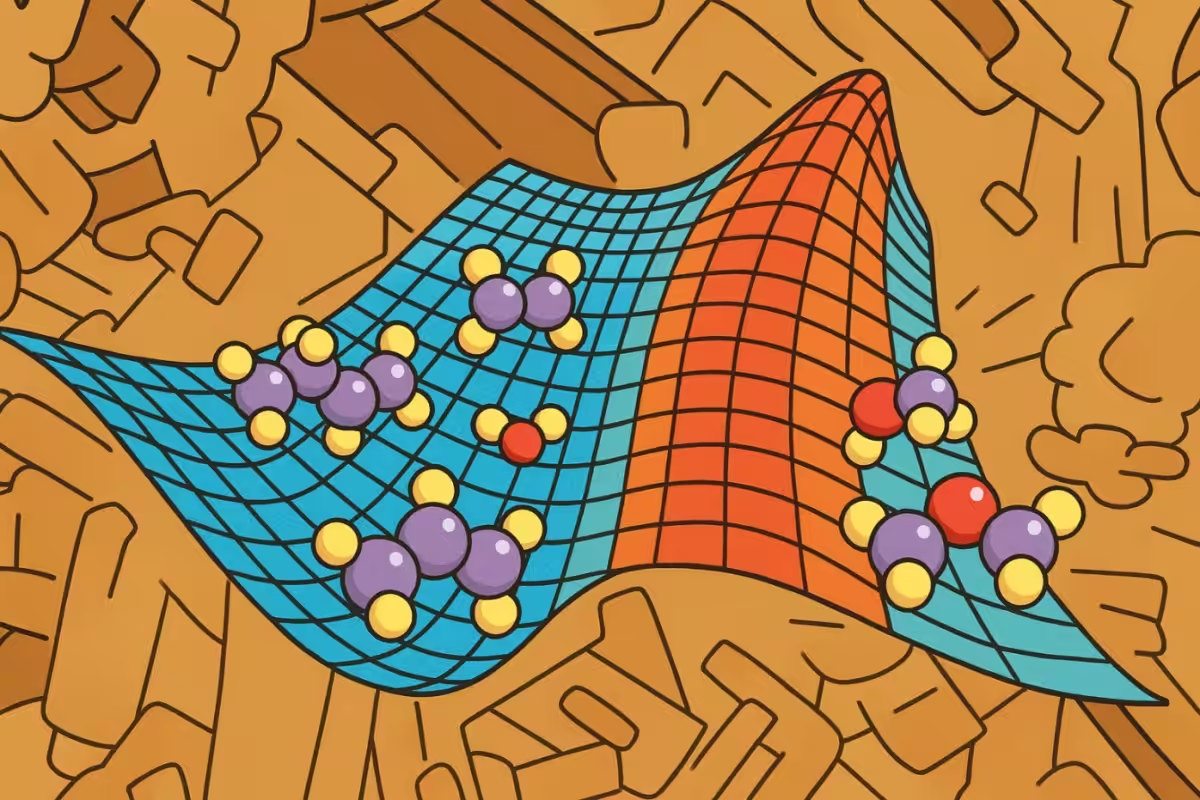
A real catalyst (background image) and a schematic of a catalytic step (foreground image). Reacting molecules (at left) acquire energy to climb the energy barrier and convert into product molecules (at right).
IMPORTANCE OF CATALYST IN BATTERIES & FUEL CELL & SUPERCAPCITOR
Batteries
Batteries are electrochemical devices that store chemical energy and convert it into electrical energy. Examples include alkaline batteries, lithium-ion batteries, and lead-acid batteries.
- Role of catalyst:
Catalysts can accelerate the electrochemical reactions that occur at the electrodes during battery operation. For example, in lithium-ion batteries, the insertion and extraction of lithium ions during charge and discharge processes can be facilitated by catalysts, leading to faster reaction rates and enhanced battery performance.
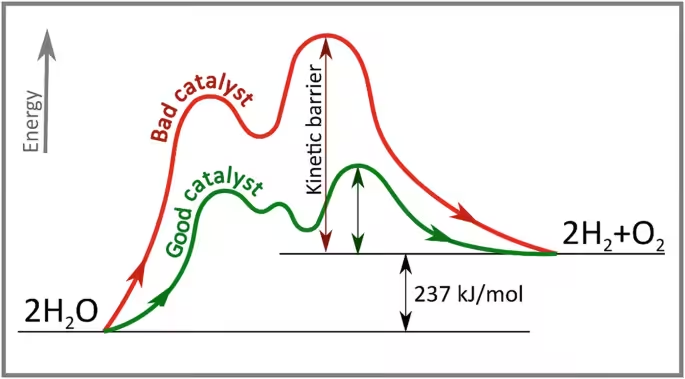 Role of catalyst scheme
Role of catalyst scheme
- Battery Lifespan:
The presence of catalysts can help minimize degradation processes within batteries, such as corrosion and side reactions, leading to longer operational lifespans. This is particularly important in lithium-sulphur and solid-state batteries, where maintaining reaction efficiency is essential
- Accelerating Reaction Kinetics
In lithium–sulfur (Li–S) batteries, catalysts expedite the conversion of lithium polysulfides, mitigating the shuttle effect and enhancing stability.
- Improving Energy Density and Reversibility
Single-atom catalysts (SACs) offer high activity and selectivity, enhancing charge/discharge efficiency and enabling higher Sulphur loadings in Li–S batteries. In Li–O₂ batteries, SACs facilitate the formation and decomposition of Li₂O₂, improving reversibility and cycle life.
- Reducing Dependence on Precious Metals
Research focuses on developing non-precious metal catalysts, such as transition metal-based materials, to replace expensive platinum group metals without compromising performance.
- Facilitating Multi-Electron Transfer Reactions
Catalysts enable complex multi-electron reactions, increasing the theoretical capacity of batteries, particularly in metal–air systems. In Li–CO₂ batteries, catalysts assist in CO₂ reduction and evolution reactions, essential for energy storage and conversion.
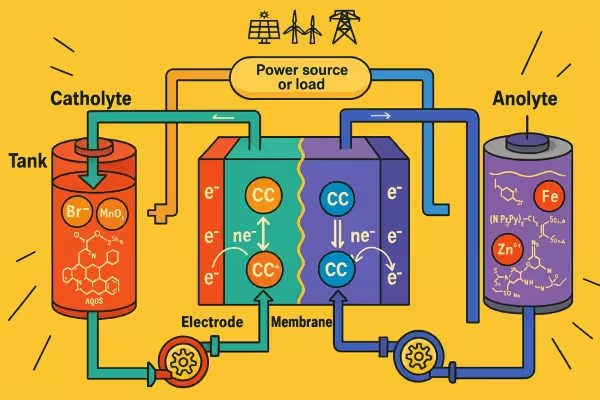
Schematic of a typical Flow of batteries during discharging
Fuel Cells
Fuel cells are electrochemical devices that convert chemical energy into electrical energy by reacting a fuel (e.g., hydrogen) with an oxidant (e.g., oxygen).

Catalyst Coated Membrane Layer
- Facilitate Key Electrochemical Reactions
Catalysts are crucial in fuel cells as they accelerate the hydrogen oxidation reaction at the anode. At the cathode, they drive the oxygen reduction reaction, completing the electrochemical process. These reactions would occur too slowly at practical conditions without catalysts.
- Increase Reaction Rates at Lower Temperatures
Catalysts reduce the activation energy required for electrochemical reactions.
This allows fuel cells to operate efficiently at lower, safer temperatures like 60–80 °C.
It eliminates the need for high-temperature setups, lowering system complexity.
- Improve Fuel Cell Efficiency
High-performance catalysts reduce energy losses caused by kinetic limitations.
They increase current density and power output by minimizing overpotentials.
Platinum-based catalysts are especially effective at enhancing ORR kinetics.
As a result, the overall efficiency and responsiveness of the fuel cell improve.
- Reduce Catalyst Poisoning
Carbon monoxide and similar species can poison platinum catalysts.
Alloying platinum with metals like ruthenium or cobalt mitigates this effect.
These alloys help oxidize CO at lower potentials, cleaning the surface.
As a result, the catalyst retains activity for longer operation periods.
Supercapacitors
Supercapacitors are electrochemical devices that store electrical energy through electrostatic double-layer capacitance and electrochemical pseudo capacitance.
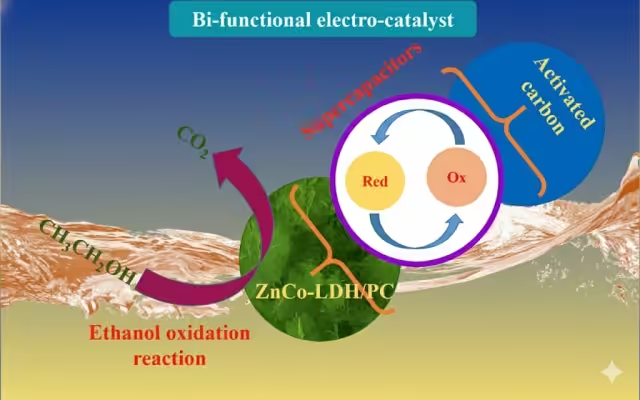
The concept of a bi-functional electro-catalyst
- Enhance Faradaic (Pseudocapacitive) Reactions
Catalysts such as transition metal oxides (e.g., Co₃O₄, MnO₂, NiO) improve charge storage through surface or near-surface redox reactions. These materials act as pseudocapacitive catalysts, storing more energy than electric double-layer capacitors (EDLCs).
- Improve Electrical Conductivity
Conductive catalysts like RuO₂, Pt, or doped carbon materials facilitate fast electron transfer. This enhances power density and allows for rapid charge–discharge cycles.
- Boost Electrochemical Stability
Catalysts help maintain structural integrity during repeated cycling. For instance, doping MnO₂ with Co or Ni improves resistance to degradation.
- Increase Surface Area and Active Sites
Nanostructured catalysts like metal-organic frameworks (MOFs) or 2D materials (e.g., MXenes) provide more active sites. This enhances interaction between electrolyte ions and electrode material.
- Enable Hybrid Supercapacitors
Catalysts play a critical role in asymmetric or hybrid supercapacitors where one electrode stores energy via Faradaic reactions. Materials like Ni (OH)₂ and Fe₂O₃ have been used effectively for this purpose.
- Support Water Splitting & Multi-functional Use
Some catalyst-integrated electrodes enable both energy storage and catalytic water splitting. This dual functionality is promising for integrated energy systems.
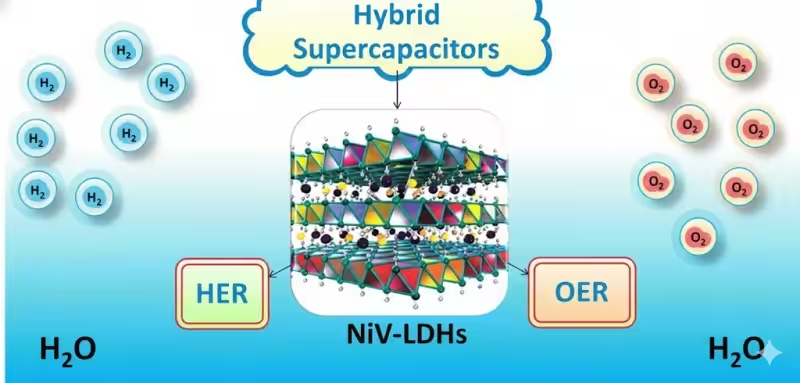
[Recent progress in water splitting and hybrid supercapacitors]
Conclusion: Future Prospects of Catalysts in Electrochemical Devices
Catalysts play a vital role in electrochemical devices, enabling efficient energy conversion and storage. Advancements in catalyst materials and design will be crucial for future breakthroughs. As research continues to progress, we can expect improved performance, sustainability, and cost-effectiveness. Catalysts will remain a key component in the development of sustainable energy technologies. Their impact will be felt across various industries, from energy storage to chemical production. With ongoing innovation, catalysts will drive us towards a more sustainable future.
References:
(Source: ACS Materials Lett. 2021, 3, 609–619)
(Source: Nature Catalysis, 2022, 5, 778–789)
(Source: Electrochimica Acta, 2023, 463, 142802)
(Source: Applied Energy, 2020, 275, 115420)
(Source: Journal of Power Sources, 2022, 543, 231932)
Applied Catalysis B: Environmental, 2022
Courtesy
Reashwanth, Sales Executive at Ultrananotech Pvt Ltd